Answer : The Density of the structure = 10.23005 g
Solution : Given,
Atomic weight of Cu = 40 g/mol
Atomic weight of Ni = 60 g/mol
Radius of Cu atom = 0.13 nm
Radius of Ni atom = 0.15 nm
Type of crystal structure = BCC
The number of atom in the BCC unit cell (Z) = 2
Avogadro's number (
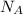 ) = 6.022 ×
) = 6.022 ×

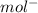
First we have to find the Edge length of unit cell i.e (a)
According to the question, in the BCC structure Ni atoms are present at the corners. So, we will use the radius of Ni atom for the calculation of edge length of unit cell. Image given below.
Edge length of unit cell (a) = 2 × radius of Ni atom = 2 × 0.15 nm = 0.30 nm = 3 ×
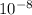 cm
cm
Converstion, 1 nm =
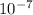 cm
cm
Volume of unit cell =
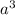 =
=
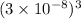 = 27 ×
= 27 ×
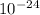
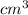
In an alloy of Cu & Ni , Cu atom is present at centre and eight Ni atom at the eight corners of the cube.
Thus, there is
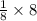 = 1atom of Ni and 1 atom of Cu
= 1atom of Ni and 1 atom of Cu
Total Molar mass of unit cell of Cu-Ni alloy (M) = molar mass of Cu + molar mass of Ni
= (40 + 60) g/mol
= 100 g/mol
Formula used :
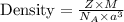
Put all the values in above formula, we get
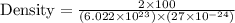
= 10.23005 g