Answer : Ionic radii of
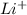 ,
,
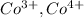 are 90 pm, 75 pm, 67 pm respectively from standard reference.
are 90 pm, 75 pm, 67 pm respectively from standard reference.
And the order of these ions from largest to smallest is
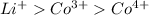 .
.
Explanation : Ionic radii is the distance away from the central atom i.e, it is the distance between the nucleus and the electron in outermost shell of an ion.
When an electron is added to an atom, forming an anion then this added electron repels the other electrons and result become an increase in the size of an atom.
Similarly, when an electron is loses from an atom, forming a cation then this loses electron attract the other electrons and result become a decrease in the size of an atom.
Ionic radii increases when we are going from top to bottom in a periodic table and decreases when we are going across the periodic table.
According to the question, the ionic radii order of the ions is
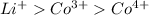 .
.
The ionic radii of the
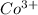 ion is larger than the
ion is larger than the
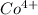 ion because as the positive charge increases on the ion then the size of the ion decreases.
ion because as the positive charge increases on the ion then the size of the ion decreases.
And in the case of
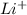 ion and
ion and
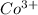 ion, the ionic radii of
ion, the ionic radii of
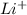 ion is greater than the
ion is greater than the
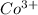 ion because as we are going across the periodic table, the size of the atom/ion decreases.
ion because as we are going across the periodic table, the size of the atom/ion decreases.