seaweed having 50 g salt in 1 L water. The bucket contains 150 g of salt in 2 L of water
amount of water present in bucket is twice to amount of water in weed
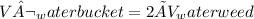
At equilibrium, volume of water in weed is x and volume in bucket is y but concentration remain same as follows:
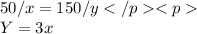
At equilibrium, weed loose z L from 1 L water to bucket containing 2 L as follows:
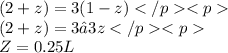
Thus, Weed will loose 0.25 L of water