The balance chemical equations are:
a.
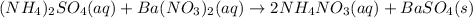
b.
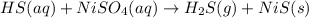
c.
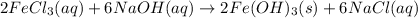
a. the balanced equation shows that the precipitate is
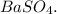
b. the balanced equation shows that the precipitate is
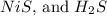 is evolved as a gas.
is evolved as a gas.
c. The balanced equation shows that the precipitate is
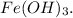
A chemical equation is a symbolic representation of a chemical reaction. It uses chemical symbols and formulas to express the substances involved in a reaction and the changes they undergo.
In a chemical equation, reactants are written on the left side of the arrow, and products are written on the right side.