Correct part is C.
Explanation: This question can be easily explained with the help of reactivity series.
The metal which lies below in the reactivity series CANNOT displace the metal which lies above it. Similarly if the metal lies above in the reactivity series, it CAN displace the metal lying low in the series.
In A part, the reaction follows
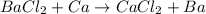
Here Ca metal lies low in the series than Ba, therefore it cannot displace Ba metal in the reaction.
In B part ,the reaction follows
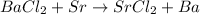
Here also Sr metal lies low in the series, therefore it cannot displace Ba.
In C part, the reaction follows
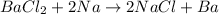
Here Na metal lies above in the series than Ba, therefore it can easily displace Ba in the reaction and will lead to form NaCl as a product.
In D part, the reaction follows
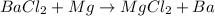
Here Mg lies low in the series, therefore it cannot displace Ba in the reaction.
You can use the image attached.