Answer:- 0.456 L
Solution:- Looking at the given information, only volume and pressure are changing and the temperature is constant. We know that, at constant temperature, the volume of the gas is inversely proportional to the pressure.
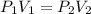
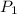 = 735 torr
= 735 torr
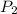 = 807 torr
= 807 torr
 = 500. mL
= 500. mL
 = ?
= ?
Let's plug in the values in the equation and solve it for final volume.
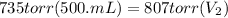
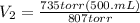
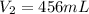
since, 1 L = 1000 mL
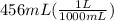
= 0.456 L
So, the volume of carbon dioxide at the new pressure will be 456 mL or 0.456 L.