Answer : The number of moles of Ti present in 5.00 moles of
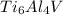 are 30.
are 30.
Explanation : Given,
Moles of
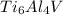 = 5.00 moles
= 5.00 moles
Now we have to determine the moles of Ti.
In
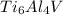 , there are 6 moles of Ti, 4 moles of Al and 1 mole of V.
, there are 6 moles of Ti, 4 moles of Al and 1 mole of V.
As, 1 mole of
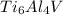 contains 6 moles of Ti
contains 6 moles of Ti
So, 5.00 mole of
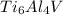 contains
contains
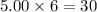 moles of Ti
moles of Ti
Hence, the number of moles of Ti present in 5.00 moles of
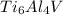 are 30.
are 30.