Answer:
One electron per single hydrogen atom.
Step-by-step explanation:
Hello!
In this case, given the reaction:
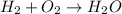
Whereas we can identify the following half-reaction for hydrogen:
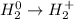
Whereas we see that each single hydrogen atom gains one electron in order to go from 0 to +1, which is also related to an oxidation half-reaction.
Best regards!