Isoelectronic species are species with same number of electrons.
The given electronic configuration of the ion is as follows:
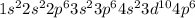
Check for the total number of electrons in the ion. The sum of number of electrons in all shells according to given electronic configuration is 36. Number of electrons in a neutral atom is equal its atomic number. According to periodic table, atomic number of krypton is 36, which is a noble gas and belongs to 18th group with electronic symbol Kr. It has the same electronic configuration thus, it is isoelectronic with the ion.
Therefore, the chemical symbol for noble gas isoelectronic with the given ion is Kr.