For triatomic gas,
 (given)
(given)
For monatomic gas,

For diatomic gas,

Let x mol of triatomic gas be added to 11 mol of monatomic gas
Internal energy of a gas is determined by:

where U is internal energy, n is number of moles,
 is specific heat capacity at constant volume, and T is temperature.
is specific heat capacity at constant volume, and T is temperature.
So, for triatomic gas, internal energy,

For monatomic gas, internal energy,

Total energy,


For mixture of gas,
 is given as:
is given as:


Substituting n = 11 + x mol
Substituting the value of
 for diatomic gas:
for diatomic gas:




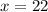
Hence,
 of triatomic gas must be added to
of triatomic gas must be added to
 of monatomic gas to get a mixture whose thermodynamic behavior was like that of a diatomic gas.
of monatomic gas to get a mixture whose thermodynamic behavior was like that of a diatomic gas.