Answer : Volume of HCl ( in ml) is 10 ml.
Solution : Given, Concentration of NaOH = 0.1 mol/L
Volume of NaOH = 20 ml
Concentration of HCl = 0.2 mol/L
Volume of HCl = ?
Formula used : Moles = Volume × Concentration
In the reaction, we see that 1 mole of NaOH react with the 1 mole of HCl.
so,
Moles of NaOH = Moles of HCl
Moles of HCl = Moles of NaOH = Volume of NaOH ( in L) × Concentration of NaOH
Converstion ml into L : 1000 ml = 1 L
Moles of HCl =
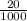 × 0.1
× 0.1
= 0.002 mol
Volume of HCl = Moles / Comcentration of HCl
=
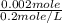
= 0.01 L
Volume of HCl (in ml) = 0.01 × 1000 = 10 ml