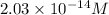First, determine the number of moles of gold.
Number of moles =
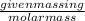
Given mass of gold =
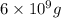
Molar mass of gold = 196.97 g/mol
Put the values,
Number of moles of gold =
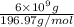
=
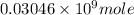 or
or
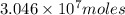
Now, molarity =
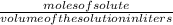
Put the values, volume of ocean =
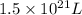
Molarity =
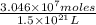
=
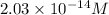
Thus, average molar concentration =