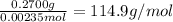Moles of NaOH required to reach the first equivalence point
=
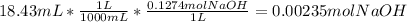
0.00235 mol NaOH is required to reach the first equivalence point when NaOH is titrated with an amino acid.
So the moles of NaOH = Moles of amino acid in the solution
Moles of amino acid = 0.00235 mol amino acid
The given mass of amino acid =0.2700g
Calculating the molar mass of amino acid from mass and number of moles: