To calculate the packing factor, first calculate the area and volume of unit cell.
Area is calculated as:
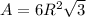
Here, R is radius and is related to a as follows:
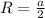
Putting the value in expression for area,
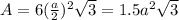
The value of a is 0.4961 nm
Since,
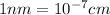
Thus,
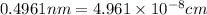
Putting the value,
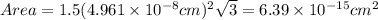
Now, volume can be calculated as follows:
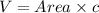
The value of c is 1.360 nm or
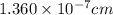
Putting the value,
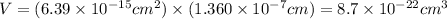
now, number of atom in unit cell can be calculated by using the following formula:
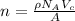
Here, A is atomic mass of
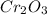 is 151.99 g/mol.
is 151.99 g/mol.
Putting all the values,
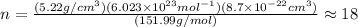
Thus, there will be 18
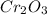 units in 1 unit cell.
units in 1 unit cell.
Since, there are 2 Cr atoms and 3 oxygen atoms thus, units of chromium and oxygen will be 2×18=36 and 3×18=54 respectively.
The atomic radii of
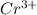 and
and
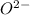 is 62 pm and 140 pm respectively.
is 62 pm and 140 pm respectively.
Converting them into cm:
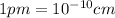
Thus,
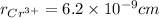
and,
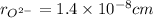
Volume of sphere will be sum of volume of total number of cations and anions thus,
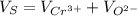
Since, volume of sphere is
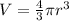 ,
,
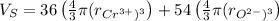
Putting the values,
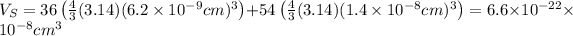
The atomic packing factor is ratio of volume of sphere and volume of crystal, thus,

Thus, atomic packing factor is 0.758.