Answer:
There are
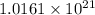 atoms of 13-C present in a 2.05 grams sample of carbon dioxide.
atoms of 13-C present in a 2.05 grams sample of carbon dioxide.
Step-by-step explanation:
Percentage of 12-C isotope = 98.93%
Percentage of 13-C isotope = 1.07%
Mass of the sample of carbon dioxide = 2.05 g
Mass of 13-C in the sample = 1.07% of 2.05 g
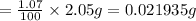
Molar mass of 13-C isotope = 13 g/mol
Moles of 13-C =

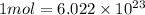 atoms/ molecules
atoms/ molecules
Atoms of 13-C in 0.001687 moles:
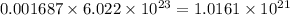 atoms
atoms
There are
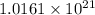 atoms of 13-C present in a 2.05 grams sample of carbon dioxide.
atoms of 13-C present in a 2.05 grams sample of carbon dioxide.