Answer: The correct option is C.
Step-by-step explanation:
Oxidation reactions are a type of chemical reactions in which a substance looses electrons and forms positive charge. The substance undergoing this reaction is being oxidized.
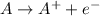
Reduction reactions are a type of chemical reactions in which a substance gains electrons and forms negative charge. The substance undergoing this reaction is being reduced.
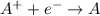
For the half reaction in which iron gains electrons means that iron is getting reduced.
Hence, the correct option is C.