Answer:
2, 8, 18
Step-by-step explanation:
The number of orbitals for any level of interest n is calculated by a formula
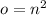 . Since each orbital contains a maximum of 2 electrons, we can calculate the total number of electrons in an nth orbital by
. Since each orbital contains a maximum of 2 electrons, we can calculate the total number of electrons in an nth orbital by
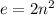 .
.
- As a result, the first energy level for n = 1 can hold a maximum of
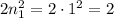 electrons,
electrons, - The second energy level can hold a maximum of
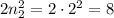 electrons,
electrons, - The third energy level can hold a maximum of
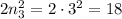 electrons.
electrons.