Option A: 109.9 L
At STP, pressure and temperature of gas is 1 atm and 273.15 K respectively.
It is given that volume at STP is 300 L, putting all the values in ideal gas equation as follows:
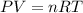
Or,
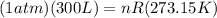
Or,
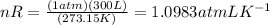
Now, volume at pressure 2 atm and T 200 K is to be calculated. Putting the temperature and pressure values in ideal gas equation,
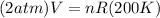
Rearranging and putting the calculated value of nR,
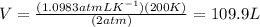
Therefore, volume of gas at 2 atm and 200 K is 109.9 L that is option A.