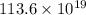Answer: The number of palladium atoms is
Step-by-step explanation:
We are given:
Mass of single palladium atom =
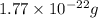
Total mass of palladium = 201 mg =
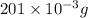 (Conversion factor: 1 g = 1000 mg)
(Conversion factor: 1 g = 1000 mg)
To calculate the number of palladium atoms, we divide the total mass of palladium to the mass of one palladium atom.
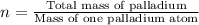
Putting values in above equation, we get:
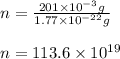
Hence, the number of palladium atoms is