The result will be 1.
Explanation: Let us assume the molecules in large scale has
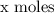 .
.
and molecules in small scale has
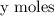
Total Composition can be expressed in terms of the mole ratio.
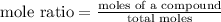
Mole ratio of molecule in large scale =
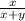
Mole ratio of molecule in small scale =
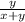
Total moles/composition will be = moles of molecules in large scale + moles of molecules in small scale
Total composition =
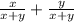
Total Composition = 1.