Answer:
10.386 grams is the mass of Cs present in 15 grams of cesium acetate.
Step-by-step explanation:
Atomic mass of cesium = 132.905 g/mol
Molecular mass of the
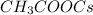 = 191.949 g/mol
= 191.949 g/mol
Percentage of an element in a compound:
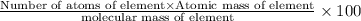
Mass percentage of cesium in 1 molecule of
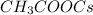 :
:
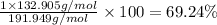
Mass of cesium in 15 grams of cesium acetate be x

x = 10.386 g
10.386 grams is the mass of Cs present in 15 grams of cesium acetate.