Answer:
Mass of C in 3.40 g of HCN =1.51 gram.
Step-by-step explanation:
Mass of sample of HCN = 7.99 g
Mass of H in 7.99 g of HCN = 0.296 g
Mass of N in 7.99 g of HCN = 4.14 g
Mass of C in 7.99 g of HCN = (7.99-0.296-4.14) = 3.554 g
Now mass of HCN = 3.40 g
Mass of C in 3.40 g of HCN =
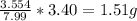
So mass of C in 3.40 g of HCN =1.51 gram.