The loss of electron from an results in the formation of cation represented by the positive charge on the element whereas gaining of electron results in the formation of anion represented by the negative charge on the element.
The alkali earth metal beryllium (
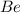 ) belongs to the second group of the periodic table. The ground state electronic configuration of
) belongs to the second group of the periodic table. The ground state electronic configuration of
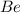 is:
is:
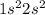
From the electronic configuration it is clear that it has 2 valence electrons in its valence shell (
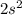 ).
).
After losing all valence electrons that is 2 electrons from
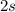 orbital. The electronic configuration will be:
orbital. The electronic configuration will be:
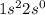
Since, lose of electron is represented by positive charge on the element symbol. So, the beryllium will have +2 charge on its symbol as
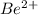 .
.
Hence, beryllium will have 2+ charge on it after losing all its valence electrons in the chemical reaction.