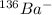In a neutral atom, the number of protons is equal to the number of electrons.
Given that the charge on the ion of the element is -1. This means that there is one electron more than the number of protons in the element.
The atomic number = Number of protons = 57 -1 = 56
Element with atomic number 56 is Barium.
Mass number of the element = Number of protons + Number of neutrons
= 56 + 80 = 136
So the identity of the species: