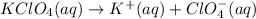Answer:- In aqueous solution, potassium perchlorate gives
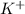 and
and
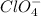 ions.
ions.
Explanations:- Potassium perchlorate formula is
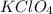 and it's an ionic compound. As per the solubility rules, all compounds of alkali metals are soluble. So, the give ions in aqueous solution. As is clear from the formula of potassium perchlorate, it gives potassium ions and perchlorate ions when dissolved in water.
and it's an ionic compound. As per the solubility rules, all compounds of alkali metals are soluble. So, the give ions in aqueous solution. As is clear from the formula of potassium perchlorate, it gives potassium ions and perchlorate ions when dissolved in water.