Iron having Z=26 with electronic configuration as
![[Ar]3d^64s^2](https://img.qammunity.org/2019/formulas/chemistry/middle-school/mc600yl4yk9w5lm7gbgboc6tlf9dqgzlut.png) has 6 electrons in the 3d shell wherein 5 electrons are aligned parallely and 1 electron as antiparallel.
has 6 electrons in the 3d shell wherein 5 electrons are aligned parallely and 1 electron as antiparallel.
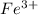 having electronic configuration as
having electronic configuration as
![[Ag]3d^5](https://img.qammunity.org/2019/formulas/chemistry/middle-school/hv2t247v5zf0lfwe6vy3gxrr2lq4itg7kh.png) has 5 electrons, all aligned in the same direction.
has 5 electrons, all aligned in the same direction.
Iron, when kept in external magnetic field, the magnetic domains of the atom get aligned in one direction that means it becomes parallel and they remain parallel even after removing the magnetic field therefore, it is considered as ferromagnetic.