Answer : The molarity of solution will be, 2.27 mole/L
Explanation :
The relation between the molarity, molality and the density of the solution is,
![d=M[(1)/(m)+(M_b)/(1000)]](https://img.qammunity.org/2019/formulas/chemistry/college/yyxcqrsbfxpi8kl5kpjvqg9p7szhpabymp.png)
where,
d = density of solution = 1.1066 g/ml
m = molality of solution = 4.666 m
M = molarity of solution = ?
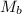 = molar mass of solute (Procaine hydrochloride) = 272.77 g/mole
= molar mass of solute (Procaine hydrochloride) = 272.77 g/mole
Now put all the given values in the above formula, we get:
![1.1066=M[(1)/(4.66)+(272.77)/(1000)]](https://img.qammunity.org/2019/formulas/chemistry/college/rtmvowd9lnootj664bmm18tweyy4uczbcc.png)
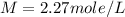
Therefore, the molarity of solution will be, 2.27 mole/L