Answer to this is O-atom.
Explanation: The Bronsted acid-base theory is the backbone of chemistry. This theory focuses mainly on acids and bases acting as proton donors or proton acceptors.
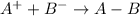
where
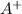 is the Lewis Acid and
is the Lewis Acid and
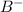 is the Lewis Base and
is the Lewis Base and
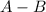 is the Covalent Bond.
is the Covalent Bond.
Reaction of dissociation of
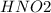 in
in
 is given as:
is given as:
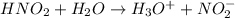
In this reaction O-atom has lone pair in water and therefore it accepts the proton from
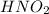 forming a Lewis Base.
forming a Lewis Base.