Answer:
Average velocity of molecules is 20 m/s and root mean square velocity of molecules is 20.24 m/s
Step-by-step explanation:
It is given that, eleven molecules have speeds 15,16,17,18,19,20,21,22,23,24,25 m/s. We have to calculate (1) average velocity (2) root mean square velocity of molecules.
Average velocity can be calculated as :
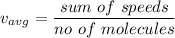
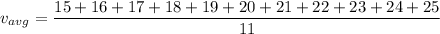
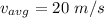
Root mean square velocity is given by :
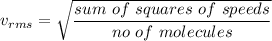
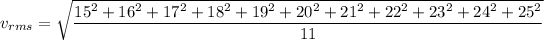
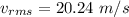
Hence, this is the required solution.