Density is the ratio of mass to the volume.
The mathematical expression is given as:
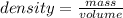
Now, density of isooctane =
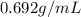
Volume =
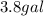
Since, 1 gallon = 3.78 L
So, 3.8 gal =
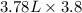
=
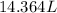
As, 1 L = 1000 mL
Therefore,
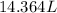 =
=

Volume in mL =
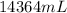
Put the values,
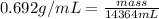
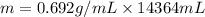
=
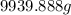
Hence, mass of 3.8 gal of the gasoline is
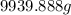 .
.