Answer : The final volume of the reaction mixture is 31.33 L
Explanation :
The given balanced chemical equation is ,
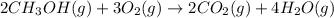
Step 1 : Find limiting reactant
In order to find the limiting reactant, we will divide the given volumes by the stoichiometric coefficient from the balanced equation. The reactant that gives lower volume is the limiting reactant.
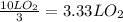
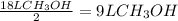
Since the volume of O₂ is lower, it is the limiting reactant.
Step 2 : Using limiting reactant, find out the volumes of product formed.
The amount of CO₂ formed can be calculated as,
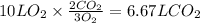
The amount of H₂O formed can be calculated as,
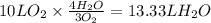
Total volume of products = 6.67 L + 13.33 L = 20 L
Step 3 : Find the volume of excess reactant.
The amount of methanol that reacts with the given amount of O₂ is
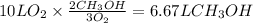
But the initial amount of methanol is 18 L.
The amount of excess reactant is 18 L - 6.67 L = 11.33 L
The total volume of gases after the reactant is complete = total volume of products + excess reactant = 20 L + 11.33 L = 31.33 L
The final volume of the reaction mixture is 31.33 L