Density is the ratio of mass to the volume. The formula of density is:
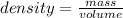 -(1)
-(1)
Density of carbon tetrachloride =
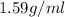 (given)
(given)
Mass of carbon tetrachloride =
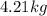 (given)
(given)
Since,
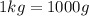
So,
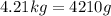
Substituting the values in formula (1):
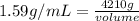
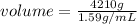
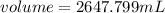
Since,
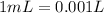
So,
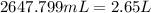
Hence, the volume of carbon tetrachloride is
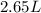 .
.