Here in the process we require
1. Heat to melt down all ice
2. Heat to raise the temperature of whole water to 100 degree C
3. Heat to boil off the water
now here for the first part
Heat required to melt the ice
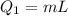
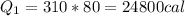
now heat required to raise the temperature to 100 degree C
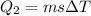
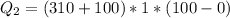
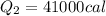
Now heat required to boil it off
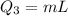
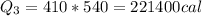
now the total heat required will be
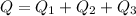
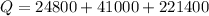
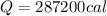
so it required 287200 calorie heat to boil it all water