Total number of atoms in the sample is the sum of number of atoms of all the elements present in the sample.
Number of gold,
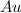 atoms in
atoms in
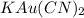 =
=
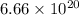 atoms (given)
atoms (given)
From the formula of compound that is
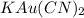 it is clear that the number of potassium and gold are same whereas those of carbon and nitrogen are 2 times of them.
it is clear that the number of potassium and gold are same whereas those of carbon and nitrogen are 2 times of them.
So, the number of atoms of each element is:
Number of potassium,
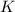 atoms in
atoms in
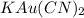 =
=
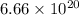 atoms
atoms
Number of carbon,
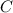 atoms in
atoms in
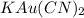 =
=
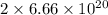 atoms =
atoms =
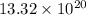
Number of nitrogen,
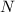 atoms in
atoms in
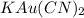 =
=
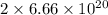 atoms =
atoms =
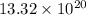
Total number of atoms in
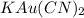 = Number of gold,
= Number of gold,
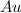 atoms+Number of potassium,
atoms+Number of potassium,
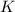 atoms +Number of carbon,
atoms +Number of carbon,
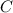 atoms + Number of nitrogen,
atoms + Number of nitrogen,
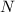 atoms
atoms
Total number of atoms in
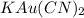 =
=
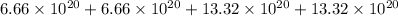
Total number of atoms in
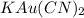 =
=
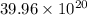 atoms
atoms
Hence, the total number of atoms in
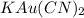 is
is
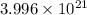 atoms.
atoms.