Energy of photon is equal to the product of Planck's constant and frequency.
The mathematical expression is given as :
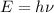
where,
E = energy of photon
h = Planck's constant i.e.

 = frequency
= frequency
Put the given values of frequency and Planck's constant in above formula, we get
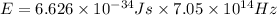
=
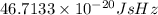
Since, Hz is equal to
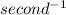
Thus, energy of photon =
 or
or
