The number of moles is given by:
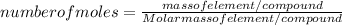
Mass of hydrogen,
 =
=
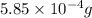 (given)
(given)
Molar mass of hydrogen,
 =
=
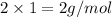
Number of moles of hydrogen,
 =
=

Reaction between
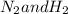 to form
to form
 is:
is:
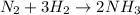
From the reaction it is clear that 3 moles of hydrogen gives 2 moles of ammonia and 1 mole of nitrogen gives 2 moles of ammonia .
So,
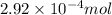 of
of
 gives:
gives:
Number of moles of ammonia,
 =
=
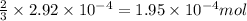
Since, in one mole of ammonia,
 there are
there are
 molecules of ammonia,
molecules of ammonia,
 .
.
So, number of molecules in
 of ammonia,
of ammonia,
 is:
is:
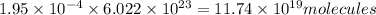
Hence, the number of molecules of ammonia produced by
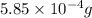 of
of
 is
is
 .
.