Answer:
The wavelength of the photon nathan would have to absorb if he wanted to jump from levels 2 to 4 = 296.53 nm
Step-by-step explanation:
The energy of the absorbed photon can be found using,
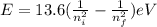 , where
, where
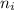 is the initial quantum level and
is the initial quantum level and
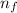 is the final quantum level.
is the final quantum level.
And we also have E =hc/λ , where h is Planck's constant, c is the speed of light and λ is the wavelength of photon absorbed.
So E is inversely proportional to λ and E is directly proportional to
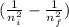
So λ is inversely proportional to
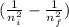
We have
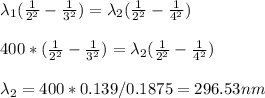
So the wavelength of the photon nathan would have to absorb if he wanted to jump from levels 2 to 4 = 296.53 nm