To calculate moles of any
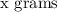 of a subtance, we need to divide the given mass by the molar mass of the substance that is
of a subtance, we need to divide the given mass by the molar mass of the substance that is
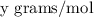 given as per the question. By simply putting the values of the two masses we will be left with the number of moles of the given substance.
given as per the question. By simply putting the values of the two masses we will be left with the number of moles of the given substance.
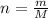
where, n= number of moles of given substance (mol)
m= given mass of the substance (grams)
M= molar mass of the given substance (grams/mol)