pH of the solution is calculated using the following formula:
![pH=-log{[H^(+)]}](https://img.qammunity.org/2019/formulas/chemistry/high-school/axmwotwj0ynnwhc73w82yohtcq8pixnkrz.png)
Here,
![[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/75vk4lm3dg3i0qv728qhnrrb5qib1loutc.png) is concentration of hydrogen ion.
is concentration of hydrogen ion.
Initial value of pH is 6.7, calculate concentration of hydrogen ion from this as follows:
![[H^(+)]=10^(-pH)](https://img.qammunity.org/2019/formulas/chemistry/high-school/ti9236ftf4agkse187j7lwj16buf5yvkle.png)
Putting the value,
![[H^(+)]=10^(-6.7)=1.9952* 10^(-7)](https://img.qammunity.org/2019/formulas/chemistry/high-school/u9ccfc5azfoy127aev0xzbwa063ubjwvqi.png)
Thus, initial concentration of hydrogen ion is
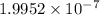 .
.
Now, final pH value is 8.7, calculate concentration of hydrogen ion as follows:
![[H^(+)]=10^(-8.7)=1.9952* 10^(-9)](https://img.qammunity.org/2019/formulas/chemistry/high-school/pv7hazmfjaqoxuohiwtmoauuoarzufe7kf.png)
Thus, final concentration of hydrogen ion is
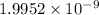 .
.
The ratio of final to initial value will be:
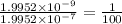
Thus, if pH increases from 6.7 to 8.7, concentration of hydrogen ion becomes
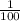 of the initial value.
of the initial value.