Answer: The mole ratio of oxygen atoms to silicon atoms in mantle is 3.6 : 1
Step-by-step explanation:
To calculate the mole ratio of oxygen and silicon in mantle, use will follow some steps:
Step 1: Converting Mass percent into mass of Oxygen and Silicon.
We are given 44.8% of oxygen is present in mantle by mass.
This means that 44.8 grams of oxygen is present in 100 grams of mantle.
Similarly, 21.5 grams of silicon is present in 100 grams of mantle.
Step 2: Calculating the moles of Oxygen and Silicon.
To calculate the number of Moles, we will use the formula:
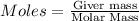
Molar mass of oxygen = 16 g/mol
Given mass of oxygen = 44.8 grams
Putting values in above equation, we get:
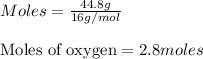
Molar mass of silicon = 28 g/mol
Given mass of silicon = 21.5 grams
Putting values in above equation, we get:
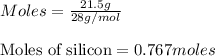
Step 3: Calculating the number of atoms of Oxygen and Silicon.
According to mole concept:
1 mole of an element contains
 number of atoms.
number of atoms.
- Calculating the number of atoms of Oxygen:
2.8 moles of oxygen will contain
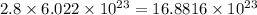 atoms
atoms
- Calculating the number of atoms of Silicon:
0.767 moles of silicon will contain
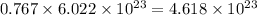 atoms
atoms
Step 4: Calculating the mole ratio of Oxygen and Silicon atoms
Mole ratio of atoms of Oxygen : Silicon =
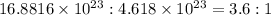
Hence, the mole ratio of atoms of Oxygen and Silicon is 3.6 : 1