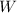 is the symbol of tungsten.
is the symbol of tungsten.
Molar mass of
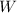 =
=
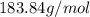
Now, number of atoms are converted to number of moles by Avogadro number i.e.

Number of moles of tungsten =
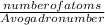
number of moles =
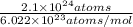
=
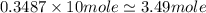
Therefore, 1 mole of tungsten consist of
 atoms
atoms
So,
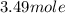 of tungsten consist of
of tungsten consist of
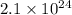 atoms.
atoms.
Number of moles is also equal to
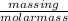
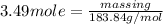
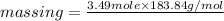
=
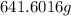 or
or
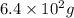
Thus, mass in g of
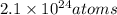 =
=
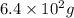 .
.