Answer:
The final volume of the gas is 112.5 mL.
Step-by-step explanation:
It is given that,
Initial volume of the gas,
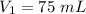
Initial pressure,
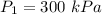
Final pressure,
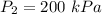
We need to find the final volume of the gas. It can be calculated using the Boyle's law as :
p v = constant
or
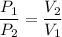 , V₂ is the final volume of the gas
, V₂ is the final volume of the gas
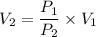
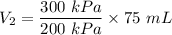
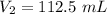
So, the final volume of the gas is 112.5 mL. Hence, this is the required solution.