pH of the aqueous solution = 9
Therefore, [H+] =
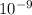 = 0.000000001 M
= 0.000000001 M
pH + pOH = 14
Therefore, pOH = 14 - pH = 14 - 9 = 5
[OH-] =
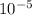 = 0.00001 M
= 0.00001 M
Concentration of OH- ions is more than H+ ions by
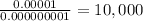 times
times
Thus, an aqueous solution at pH 9 has free OH-ions 10,000 times more than free H+ ions.