Density of any substance is mass of the substance per unit volume. Density, d=m/V, where m is mass of the substance and V is the volume occupied by the substance. The amount of water displaced by an object when it is submerged in water equals the volume of the object. So, due to submerge of piece of zinc alloy in water present in graduated cylinder, water level rises. The increase in the volume of water is the volume of the zinc piece.
Volume of water in graduated cylinder before addition of zinc piece (v₁)= 20 ml, Volume of water in graduated cylinder after addition of zinc piece (v₂)= 23.2 ml, Rise in the volume due to submerge of the piece = (v₂ - v₁)ml= 23.2-20.0= 3.20 ml. So, volume of the piece is 3.20 ml and mass of the zinc piece is 25.0 g.
Hence density zinc alloy is equal to
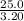 g/ml= 7.81 g/ml= 7.8 g/ml (expressed in two significant figure).
g/ml= 7.81 g/ml= 7.8 g/ml (expressed in two significant figure).