Answer:
The cations are
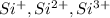
Step-by-step explanation:
Atomic number = Number of protons present in the nucleus of an atom of the element
Number of protons =14
Atomic number of an element will be 14.
The name of the element with 14 atomic number is silicon. It has equal number of electrons. Since an atom is a neutral species.
After loosing electrons atom gain positive charge.
![[Si]=1s^22s^22p^63s^23p^2](https://img.qammunity.org/2019/formulas/chemistry/middle-school/174iszlns8065bd2hfyd7cz0kr8ku1a5yh.png)
After losing an electron is becomes

![[Si^+]=1s^22s^22p^63s^23p^1](https://img.qammunity.org/2019/formulas/chemistry/middle-school/ui3uo5c01f4a4gt28tc8u4os4t6bmhu3cd.png)
After losing 2 electron is becomes

![[Si^(2+)]=1s^22s^22p^63s^23p^0](https://img.qammunity.org/2019/formulas/chemistry/middle-school/h2nr67oiz48axz9bn7olamdsua7ho4baa2.png)
After losing 3 electron is becomes

![[Si^(3+)]=1s^22s^22p^6](https://img.qammunity.org/2019/formulas/chemistry/middle-school/rorv4au2udaxrvhj0bo27nit431vy15pqu.png)