Mass of each silver coin = 5.67 g
Molar mass of silver = 107.87 g/mol
Calculating the moles of silver from given mass and molar mass of silver:
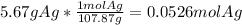
Calculating the atoms of silver in 0.05260 mol using the conversion factor,
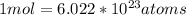 :
:
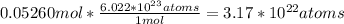
Therefore,
 atoms of silver are present in each coin.
atoms of silver are present in each coin.