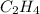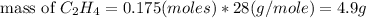Definition : Limiting reagent is the substance in a chemical reaction that is consumed when chemical reaction is complete.
step 1 : first write the chemical reaction and then balanced the chemical equation
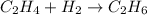
step 2 : convert given masses into moles.
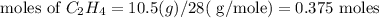
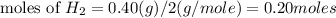
step 3 : Now find the limiting reagent.
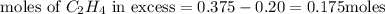
Now we conclude that
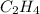 is the limiting reagent.
is the limiting reagent.
step 4 : Amount of the excess reactant i.e