Nitrogen combine with hydrogen to produce ammonia
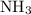 at a
at a
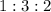 ratio:
ratio:
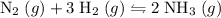
Assuming that the reaction has indeed proceeded to completion- with all nitrogen used up as the question has indicated.
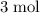 of hydrogen gas would have been consumed while
of hydrogen gas would have been consumed while
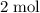 of ammonia would have been produced. The final mixture would therefore contain
of ammonia would have been produced. The final mixture would therefore contain
Apply the ideal gas law to find the total pressure inside the container and the respective partial pressure of hydrogen and ammonia: