Answer : Average molecular weight of group A = 104.0276 g
Average molecular weight of group B = 104.0920 g
Solution : Given, Moles of group A styrene = 5 moles
Moles of group A benzoyl peroxide = 0.0010 moles
Moles of group B styrene = 1.5 moles
Moles of group B benzoyl peroxide = 0.0010 moles
Molecular mass of styrene = 104 g/mole
Molecular mass of benzoyl peroxide = 242.23 g/mol
Formula used : Average molar mass = Total mass / Total moles
For group A,
Average molar mass of group A =
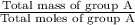
=
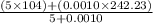
=
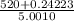
=
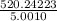
= 104.0276 g
Average molar mass of group A = 104.0276 g
For group B,
Average molar mass of group B =
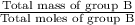
=

=
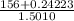
=
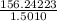
= 104.0920 g
Average molar mass of group B = 104.0920 g