The chemical reaction in which number of atoms of each element present in the reactant side is equal to the number of atoms of that element in product side, such reactions are said to be a balanced chemical reaction.
The chemical symbol for sodium is
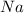 .
.
The chemical symbol for fluorine gas is
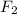 .
.
The chemical symbol for sodium fluoride is
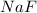
The sodium fluoride is prepared from the reaction between sodium metal and fluorine gas can be written as:
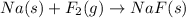
The above reaction is not balanced as the number of fluorine atoms are not same on reactant and product side. So, in order to balance the reaction we will multiply
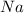 with 2 on reactant side and
with 2 on reactant side and
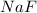 with 2 on product side. Thus, the balanced reaction will be:
with 2 on product side. Thus, the balanced reaction will be:
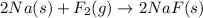
Thus, the balanced chemical equation is
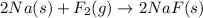 .
.